Founded in 1996 in Shenzhen, China, Headway is currently the leading manufacturer & supplier of H. Pylori diagnostic—13C & 14C urea breath test—systems in the world. We are committed to making it easier to pursue good health by leveraging our more than 25 years of innovation in breath tests.
We have 3 R&D Centers,3 Manufacturing Centers in 70,000 m2 Production Area.We are serving in 16 Countries & Regions and 20,000+ Medical Institutions for 300 Million+ People.
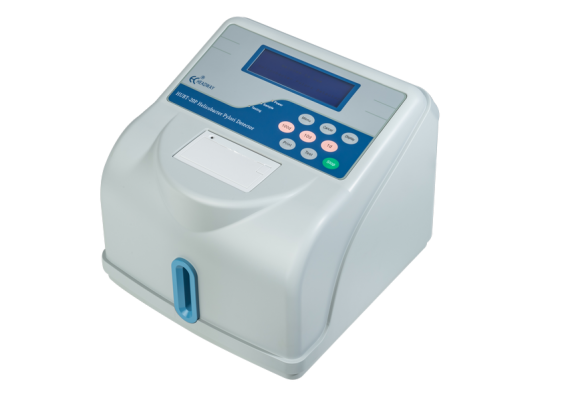
Headway HUBT-20P is our major achievement after many years of system improvement. H. Pylori infection can be accurately
detected using this 14C-UBT system which tracks the 14C-labeled isotope in the breath exhaled by the patients,thus providing excellent medical support for patients and doctors.
- The tolerance of relative variation of 14C detection efficiency is ≤30% after operating for successive 48 hours
- The efficiency of detection on 14C unquenched standard source is ≥15%
- The background count of 14C is ≤50CPM
- A fully-automated design enables easy, automatic measurement without pushing any buttons.
- The detector allows automatic fault diagnosis and correction.
- The detector can be either operated alone or connected to a PC. It has a built-in printer for automatic printing.
- The detector is connected to the local area network of the hospital and can achieve interconnection.
- The innovative design of a cartridge and a disposable mouthpiece makes UBT more convenient. Just one breath is needed to detect H. Pylori infection.

Test Procedure
- The patient takes a 14C-urea capsule
- Wait for 15 min
- The patient exhales on to the card without inhaling, until the card's color changes from orange to yellow
- Use the HUBT-20P test collection card for the diagnosis (positive/negative)

